In organic reactions, the gain or loss of electrons is not obvious, contrary to inorganic reactions. We think of oxidation and reduction as...
In organic reactions, the gain or loss of electrons is not obvious, contrary to inorganic reactions.
We think of oxidation and reduction as the gain or loss of elements in a product.
For example,
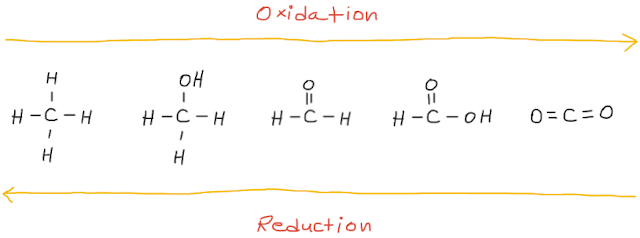
If the reaction increases the number of C-X bonds (where X = is an element more electronegative than carbon, usually oxygen or a halogen) or the number of C-H bonds decreases in the product, then it is an oxidation reaction.
On the other hand, if the reaction decreases the number of C-X bonds (where X = is an element more electronegative than carbon, usually oxygen or a halogen) or the number of C-H bonds increases in the product, then it is a reduction reaction.
Also, we can look at the reagent used in the reaction. If the the reagent is an oxidizing agent (e.g. O2 or Br2) or a reducing agent (e.g. H2, NaBH4), it is an oxidation reaction or a reduction reaction, respectively.
Keep in mind that in an oxidation-reduction reaction you always have a species that is oxidized and other that is reduced.
General schemes for the oxidation and reduction of a carbon compound are shown below.
![[headerImage]](https://blogger.googleusercontent.com/img/b/R29vZ2xl/AVvXsEigO5KyLhyphenhyphendr9pDr7KsROfd8UclK9W8ukZsUduYj6g3HCArd4bkm58exk1NgXPzrMPNfdkoWbVCsviGSi1ZFdQWs_E46VTqFXRtD2-8nHt_h90l2bpUOpxwcNm0WgHBouLdQCO0ewq8xUI/s16000/Screen+Shot+2021-04-27+at+12.44.15+AM.png)